Menu
Podiatry Tools
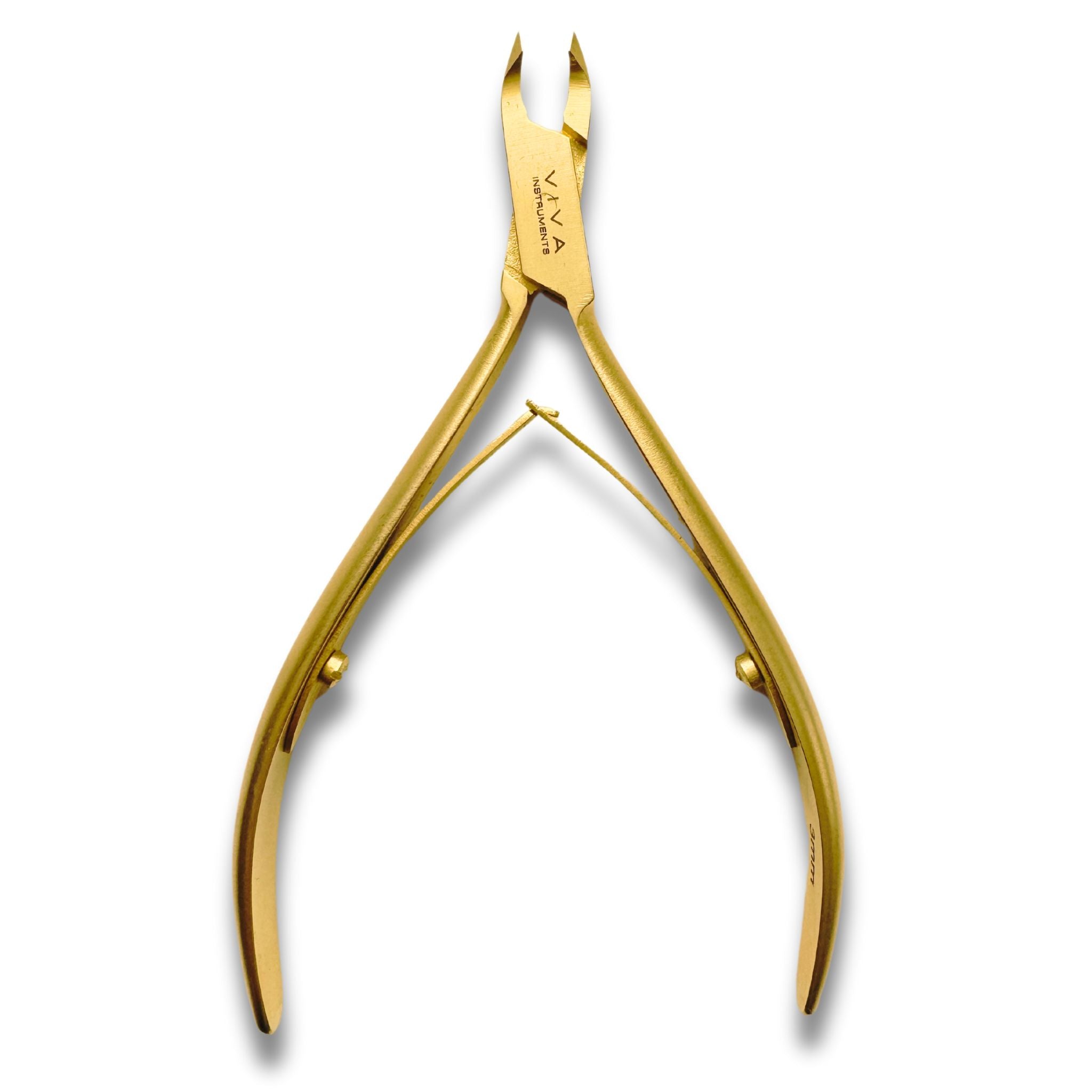
Cantilever Nippers CuttersToenail Nippers CuttersIngrown Nail Nippers Cuticle Nippers Tissue Forceps Artery Forceps Titanium Instruments Scalpel Blade Handles Scissors Bandage Applicators Files & RaspsBlacks File & ProbesNail Elevators & Spatulas Instruments Sets & KitsInstrument Trays Diamond Nail Drill BitsCarbide Tungsten Bits Ceramic Nail Drill BitsSanding Discs & Bands BULK BUY Maintenance
Manicure & Pedicure Tools
Eyelash Tweezers
Manicure & Pedicure Tools Nippers | Scissors | Files & Drill Bits


Manicure & Pedicure Tools Nippers | Scissors | Files & Drill Bits
-
Best Sellers
-
Categories
Manicure & Pedicure Tools Nippers | Scissors | Files & Drill Bits

Manicure & Pedicure Tools Nippers | Scissors | Files & Drill Bits
Account
Sign in
Cart
0
$0.00
-
Home
-
Shop
Podiatry Tools
- Cantilever Nippers Cutters
- Toenail Nippers Cutters
- Ingrown Nail Nippers
- Cuticle Nippers
- Tissue Forceps
- Artery Forceps
- Titanium Instruments
- Scalpel Blade Handles
- Scissors
- Bandage Applicators
- Files & Rasps
- Blacks File & Probes
- Nail Elevators & Spatulas
- Instruments Sets & Kits
- Instrument Trays
- Diamond Nail Drill Bits
- Carbide Tungsten Bits
- Ceramic Nail Drill Bits
- Sanding Discs & Bands
- BULK BUY
- Maintenance
-
On Sale
-
3 For 2 & Bundles
-
New Arrivals
- £5 OR Less Sale
-
Info